
Sana Biotechnology
We could deliver any biotherapeutic payload to any cell in the body?
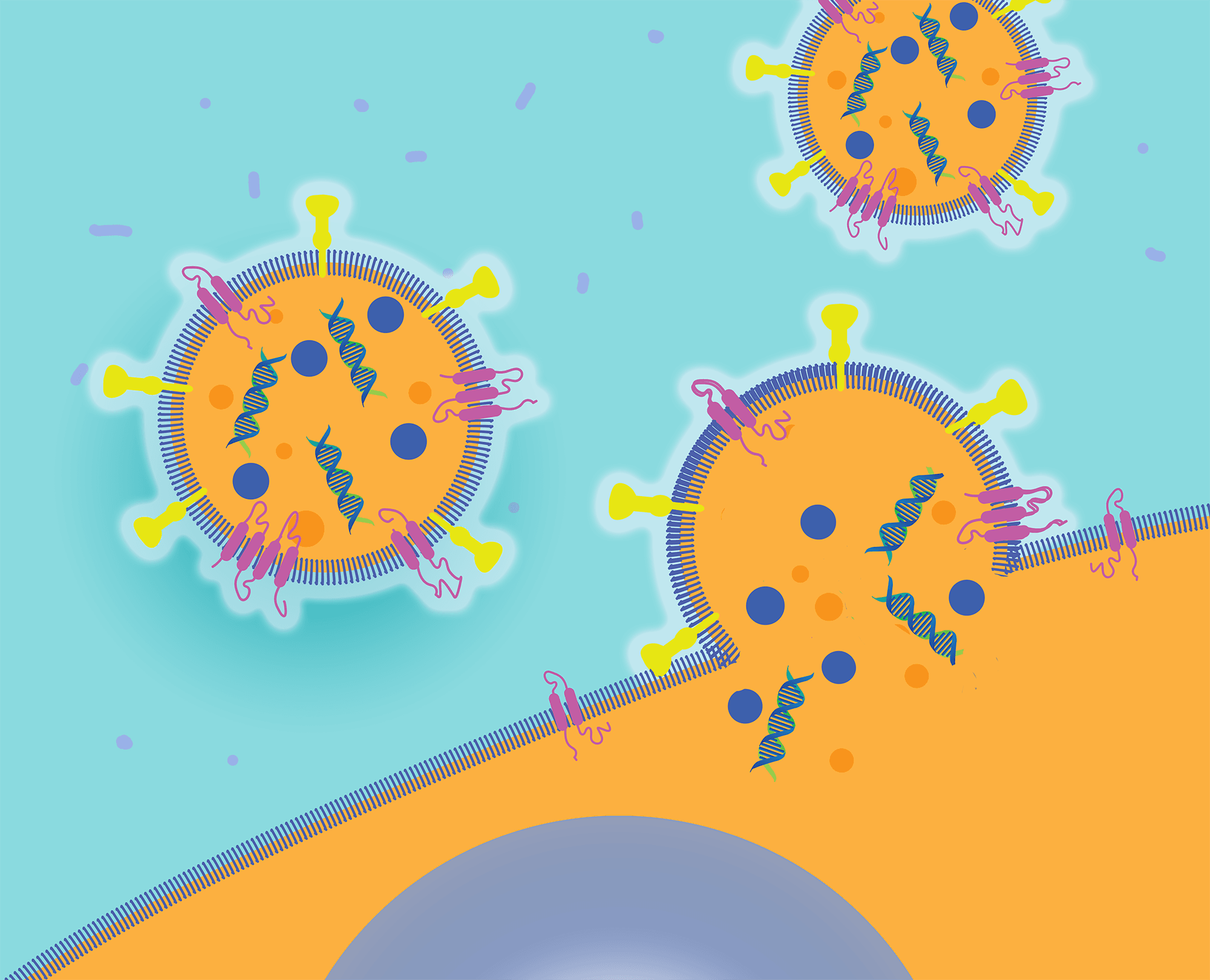
We can efficiently deliver protein and nucleic acid therapeutics into specific cells by engineering proteins called fusogens.
Almost every human disease originates within cells. Yet the only therapeutics with easy access to the cellular cytoplasm are small molecules. Small molecules were the foundation of the modern drug industry from its earliest days, but their relative simplicity means they cannot permanently cure or reverse most diseases. In contrast, proteins and nucleic acids are sophisticated molecules that offer dramatic therapeutic outcomes—as breakthroughs in the fields of gene editing, gene therapy, RNA interference, and mRNA therapy have demonstrated. But it’s hard for protein and nucleic acid therapies to reach their targets inside cells. In an ideal world, we would combine the therapeutic power of protein and nucleic acid drugs with the delivery breadth of small molecule drugs. To realize that goal, we need breakthroughs in intracellular delivery.
Focus
Intracellular delivery faces two fundamental challenges: first, finding the specific target cell type among all the cell types in the body; and second, efficiently overcoming the thermodynamic barrier of depositing hydrophilic payload molecules across the hydrophobic cell membrane. Intracellular delivery technology looks much the same today as it did in 2010. Most approaches are based on synthetic-chemistry-derived cationic and ionizable lipid nanoparticles. These nanoparticles transiently pop the cell’s endosome in order to release their cargo into the cell’s cytoplasm. Despite decades of investment, this approach has yet to achieve high cell specificity or efficient cytoplasmic delivery. This is because lipid nanoparticles are almost indiscriminately engulfed by a limited number of highly endocytic cell types (for instance, macrophages) and are capable of releasing only about 1% of their nucleic acid cargo.
But life wouldn’t be possible without the highly efficient flow of proteins and nucleic acids across membranes. Trillions of times every second, complex molecules travel from the nucleus to the organelles, between organelles, from organelles to the cell surface, and from one cell to another. Clearly, biology has evolved a solution to efficiently and specifically deliver molecules that synthetic chemistry has yet to discover.
Origins
In 2016, a founding team at Flagship Labs, led by General Partner Geoffrey von Maltzahn, PhD, and Principal Jacob Rubens, PhD, began a series of experiments that explored whether biology’s solution to intracellular delivery could be applied to nucleic acid and protein drugs. The researchers were inspired by remarkable feats of cytoplasmic exchange between cells, including instances where cells appear to exchange proteins, nucleic acids, and even entire organelles such as mitochondria with one another.
The exchanges occur in a variety of ways: Cells secrete lipid bilayer membrane vesicles with complex payloads, including exosomes and microvesicles, which bind to their payload and deliver it to the cytoplasm of target cells. Cells also form tunneling nanotubes to directly connect their cytoplasm to their neighbors’, creating a cytoplasm-exchange superhighway. Cells even fuse to form complex syncytia, such as in skeletal muscle, where multiple cells come together to share their entire cytoplasm.
The precursor to Sana, a Flagship Protoco, (prototype company) named FL39, examined how this extraordinary biology works to discover whether it could be harnessed to deliver therapeutic payloads. Given how prevalent biomolecular transport is across membranes in our bodies, the founding team wondered, “What if we could deliver any biotherapeutic payload to any cell in the body?” It turns out that intracellular delivery is enabled by a unique category of membrane proteins called fusogens. These fusogens can be found on the surface of membrane vesicles and enveloped viruses that bind to and fuse to target cells, delivering their payload into the cell cytoplasm. Fusogens are essential to life: The 2013 Nobel Prize was awarded for seminal work uncovering the critical role that fusogens play in intracellular biology, where vesicles programmatically fuse with one another, allowing entire repertoires of biomolecules to specifically and efficiently enter another membrane-enclosed region of the cell.
Breakthrough
Fusogens function as remarkable mechanical enzymes that can overcome the repulsive forces of bringing two lipid membranes together and achieve close to 85% efficiency in membrane fusion, making them over 100 times more efficient than cationic lipids. In addition, fusogens can have exquisite cell specificity, fusing only to cells that display a target ligand, such as a surface receptor, lipid, or sugar. Enveloped viruses, like SARS-CoV-2, HIV, and measles, rely on fusogens to seek out and deliver their genome only to their target cells. The founding team, using a combination of bioinformatics and primary searching, built the first proprietary database of more 20,000 fusogens across all functions and branches of the tree of life, and they realized that fusogens could be modularly reprogrammed to potentially target any cell type in the human body. They hypothesized that by harnessing fusogens they might be able to solve the intracellular delivery challenge.
Later in 2016, FL39 was renamed Cobalt Biomedicine. Its mission was to develop Fusosome Therapeutics™: the first biomedicines to unlock the full potential of intracellular therapies. These consist of two parts: 1) a therapeutic effector in the vesicle lumen, such as a gene therapy, gene-editing protein, mRNA, or other specific biomolecules, and 2) an engineered fusogen on the vesicle surface that targets and catalyzes fusion with a specific cell type. Cobalt built a synthetic biology platform that directs specific molecular payloads to the Fusosome lumen and decorates the vesicle’s surface in any fusogen. In addition, Cobalt developed a plug-and-play technology to rationally target a fusogen to any cell-surface receptor. Remarkably, this approach allows nearly perfect tropism by making the activity of fusogens contingent upon their binding to specific receptors, with little to no activity following nonspecific engulfment by macrophages and other cell types. This new category of medicines is able to convey biotherapeutics directly into the cytoplasm, bypassing the challenge of endosome escape that significantly decreases the potency of lipid nanoparticles. And it can fuse to cells in a receptor-specific fashion, even bypassing the liver’s reticuloendothelial system that gobbles up other delivery technologies.
Advantage
By the middle of 2018, the Cobalt team had grown to more than 35 people. They discovered and engineered fusogens that deliver their payload to five cell types with specificity that is orders of magnitude greater than that of other technologies such as lipid nanoparticles. In addition, they showed that Fusosomes were capable of delivering many different types of intracellular payloads, including gene therapies, gene-editing proteins, RNA, and even entire organelles. With this evidence in hand, Cobalt was confident that it could build a Fusosome to enable specific and efficient delivery of diverse biotherapeutic payloads to any cell to treat a wide range of human diseases. The team began to search for executives sufficiently ambitious to realize this vision.
Von Maltzahn and Rubens knew they had found the entrepreneurial team that the development of Fusosome Therapeutics demanded when they began talks with Sana Biotechnology’s CEO Steve Harr, Chairman Hans Bishop, Executive Vice Chairman Richard Mulligan, and others at Sana. The Sana team was assembling a portfolio of stem-cell, immune-silencing, and manufacturing technologies that were complementary to Cobalt’s platform. Cobalt and Sana officially merged in early 2019, under the Sana name, with the goal of creating a first-in-category therapeutics company that has the integrated expertise to repair or replace any cell in the body, and with the research, development, and manufacturing capabilities to allow both in vivo and ex vivo engineering of cells.
Today, Sana employs more than 200 people at sites in Cambridge, Mass., Seattle, and San Francisco. The company possesses the scientific knowledge, creativity, rigor, and know-how to transform medicine by treating diseases at their origin: inside cells.
